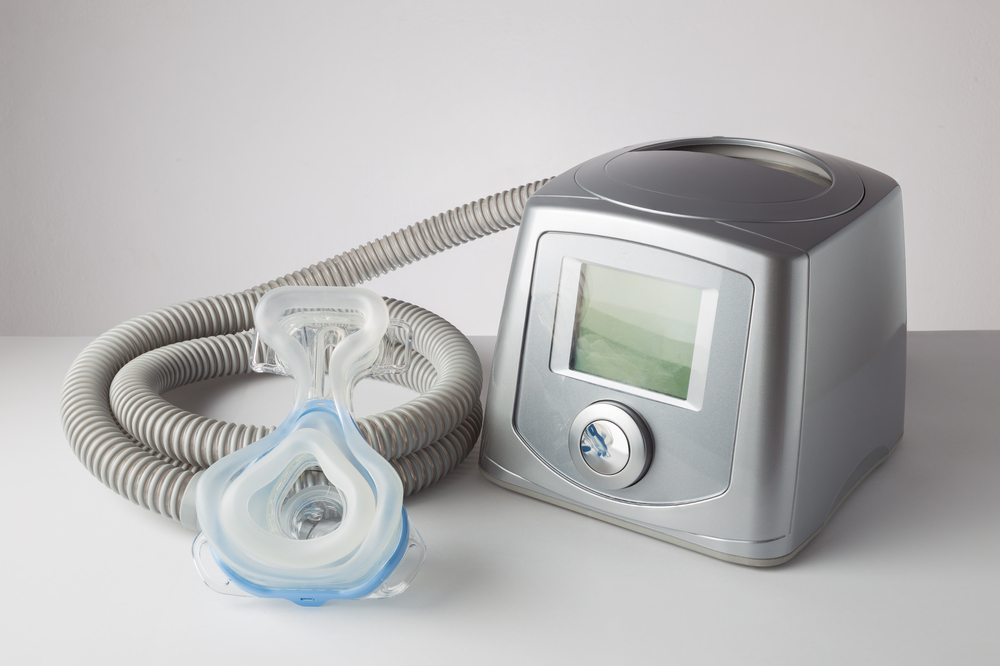
More Ventilators Recalled by Philips Respironics
Philips Respironics has issued a new recall for thousands of Philips Trilogy Evo ventilators. More than 70,000 ventilators have been recalled over concerns that dirt and debris may collect in the breathing tubes and restrict airflow. This is the second recall issued for Trilogy Evo ventilators and has been associated with two injuries and one death.
The latest recall for Philips Respironics ventilators covers several models of the Trilogy Evo line of devices distributed between March 2019 and March 2023.
Recalled devices include:
- Trilogy Evo
- Trilogy Evo O2
- Trilogy EV300
- Trilogy Evo Universal
- Garbin Evo
- Aeris EVO
- LifeVent EVO2
The most recent recall has been designated as Class I by the U.S. Food and Drug Administration (FDA). A Class I recall indicates that malfunction of the device may result in serious harm or even death. The FDA has already received over 500 reports of malfunction, including two associated injuries and one death.
Dust and Debris Accumulation in Trilogy Evo Ventilators
Philips Respironics Trilogy Evo line of ventilators can provide continuous positive airway pressure (CPAP) or intermittent positive airway pressure in people who need mechanical ventilation. They are used in both children and adults in hospitals, healthcare settings and in transport of patients needing airway support. One of the devices, the Trilogy EV300, is also available for home use.
The recall was issued for Trilogy Evo ventilators after some devices were found to have dust and debris from the environment collecting in the air tubes. Debris collection has the potential to buildup and block air vents, resulting in decreased or improper air pressure or air volume. This may result in the patient not receiving enough oxygen or cause build up of carbon dioxide which can lead to serious injury or death.
Prior Recalls by Philips
The Philips Trilogy Evo recall is only the latest in a long string of recalls issued by Philips for multiple ventilators and other breathing devices including CPAP and BiPAP machines. A prior Class I recall for some Trilogy Evo ventilators was issued for internal sensor issues. The sensor may have overreported the amount of oxygen being delivered which could lead to under-delivery of oxygen.
In 2021, Philips recalled about 5.5 million CPAP and BiPAP devices due to possible degradation of sound abatement foam. The degradation could result in particle release and toxic outgassing which could cause harm to patients. A number of these ventilators underwent repair, however in November 2022, a new recall was issued.
The reworking of the respirators was found to have a potential displacement of new sound abatement foam which could become dislodged and block airflow. The devices may also have contained particulate matter in air pathways. Philips now claims that 98% of the ventilators have been repaired or replaced. The most recent recall is currently estimated to affect 70,000 ventilators in the U.S. or about 114,000 ventilators worldwide.
News Tags
- urgent
Have you been affected by a drug or device listed?

You May Be Eligible for Compensation.
If you or a loved one suffered injury or death after using a drug or medical device, contact us today for a free case evaluation.
We value your privacy. By submitting this form, you agree to our privacy policy and disclaimer.