Philips CPAP Recall Severity Upgraded by FDA
*We are no longer accepting cases*
A recent recall of Philips CPAP and ventilator devices has been upgraded to Class I by the U.S. Food and Drug Administration. Class I is the most severe type of recall which indicates that a malfunctioning medical device may cause serious health problems or result in death.
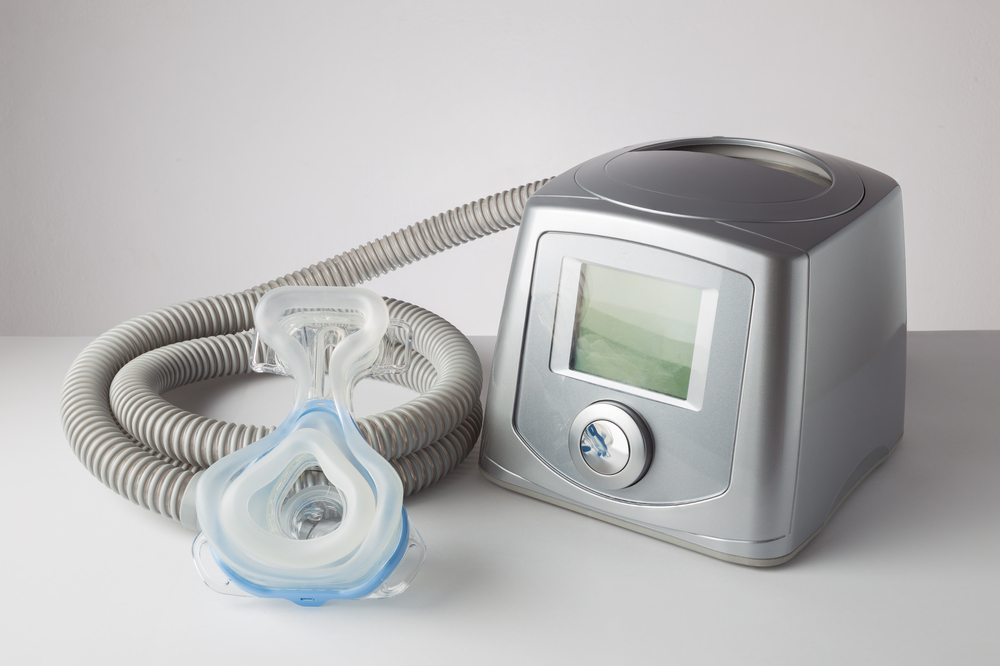
The Philips recall was issued June 30, 2021, for multiple models of CPAP, BiPAP and Ventilators made by Philips Respironics. It was initially issued as an unclassified recall, only as a safety notification but has now been officially designated Class 1. The recall involved millions of sleep apnea and ventilator devices. Though Philips has reported no injuries that have occurred, with the Class 1 announcement, the FDA has said that over 100 injuries and 1,200 complaints have been reported for products included in the CPAP/Ventilator recall.
Philips began pulling some of its sleep apnea and ventilator devices from the U.S. market on June 14, 2021, after revealing that some of the devices may pose a risk of exposure to polyester-based polyurethane (PE-PUR) which may breakdown during use. The PE-PUR may degrade into particles which can be ingested and may out-gas chemicals that may be inhaled. An official safety notification was announced by the FDA on June 30, 2021.
Polyester-based polyurethane foam is used as part of many of Philips sleep apnea CPAP and ventilator devices as a sound dampening or sound-abatement component. It has been found to be at risk of degrading or off-gassing, particularly under high heat, high humidity and under unapproved cleaning methods such as ozone cleaning.
PE-PUR exposure may result in difficulty breathing, lung injury, toxic reactions and may increase the risk of cancer. Exposure may cause immediate or longer-term symptoms such as headache, throat irritation, coughing, nausea and vomiting and more serious effects.
Philips has indicated that the recall may affect three to four million devices worldwide, with half of those in the U.S. and mainly involving the first-generation DreamStation CPAP products used to treat sleep apnea. Both Philips and the FDA have stated that people using the devices for non-life sustaining purposes are advised to contact physicians before discontinuing use.
Notwithstanding claims relating to this product, the drug/medical device remains approved by the U.S. FDA.
Source:
News Tags
- urgent
Have you been affected by a drug or device listed?

You May Be Eligible for Compensation.
If you or a loved one suffered injury or death after using a drug or medical device, contact us today for a free case evaluation.
We value your privacy. By submitting this form, you agree to our privacy policy and disclaimer.